Taking an Artificial Leaf Out of Nature’s Book March 13, 2010
Posted by calvinus in Energy, Nano, Renewables, Solar.Tags: Energy, Nano, Renewables, Solar
add a comment
Solar cells are common enough. Small solar cells are found in a variety everyday applications. The normal example I give is in calculators although it looks like these are becoming less and less common if my students are anything to go by – many of my first years don’t own a “proper” calculator – iPhones are the order of the day. However, plonk a solar cell on the side of a building and we have an additional problem. Buildings tend not to move, but the sun does which means you are limited practically to south-facing surfaces. Now, a full GIS study of how to maximise the power harvested by solar cells in a built-up area would help overcome some of this problem, but it doesn’t solve another issue. Dirt!
Would you eat your dinner off the street? Stick something in an urban environment and you will inevitably get all manner of dust, detritus and general keech stuck to it. A layer of grime on your shiny solar cells means light will not make it through to the photovoltaic underneath, which means a less efficient solar cell. Yi Cui at Stanford University has come up with an elegant way around this dirty issue.

What gives lotus leaves their water repelling properties are tiny spikes along the surface of the leave which stops water droplets from wetting the surface. Given that most dust particles are deposited on glass surfaces after rainfall, Cui figured it would be useful to put this to good effect in solar cells. By pattering the amorphous silicon that solar cells are often made from in a way that mimics the lotus leaf, water droplets can run off the surface taking any dirt with them, rather than sticking around and evaporating (which would leave the dirt behind)[1]
deposited on glass surfaces after rainfall, Cui figured it would be useful to put this to good effect in solar cells. By pattering the amorphous silicon that solar cells are often made from in a way that mimics the lotus leaf, water droplets can run off the surface taking any dirt with them, rather than sticking around and evaporating (which would leave the dirt behind)[1]
There is an additional, more fundamental benefit to this type of patterning. This films of amorphous silicon tend to be pretty flat. They also have a habit of behaving a little too much like a mirror than is useful for a solar cell. If you absorb light, you generate electricity; if you reflect light, like a mirror, you lose efficiency. The roughened “domed” structure means that less light is reflected. Cui saw this in their results – their rough structured cells were found to be 5.9% efficient, which is better than the 4.7% efficiency for flat, smooth surfaces. Sometimes it pays to take the rough with the smooth.
![]() [1] Zhu, J., Hsu, C., Yu, Z., Fan, S., & Cui, Y. (2009). Nanodome Solar Cells with Efficient Light Management and Self-Cleaning Nano Letters DOI: 10.1021/nl9034237
[1] Zhu, J., Hsu, C., Yu, Z., Fan, S., & Cui, Y. (2009). Nanodome Solar Cells with Efficient Light Management and Self-Cleaning Nano Letters DOI: 10.1021/nl9034237
Recharge Your Batteries November 15, 2009
Posted by calvinus in Batteries, Energy, Physical Chemistry, Renewables.Tags: Batteries, Energy, Physical Chemistry, Renewables
2 comments
![]() Every so often, you come across an article that looks really interesting. Interesting in a good way . Often you get the chance to review manuscripts that are “interesting”, but this is not one of those.
Every so often, you come across an article that looks really interesting. Interesting in a good way . Often you get the chance to review manuscripts that are “interesting”, but this is not one of those.
I was actually looking for something else and in the process of re-establishing some order to the chaos, this Chemical Communications article popped up from the pile. Renewable electrochemical systems, i.e., rechargeable batteries, have long undergone development and if I compare my first rechargeable batteries with the lifetime of the battery in the laptop I am using at the moment, the difference is night and day. Having said that, such efficiencies may partly bedown to better power management. The underlying technology is not that radically different. As Jean-Marie Tarascon commented at a meeting at the Royal Society in London at the start of this year, rechargeable battery research has advanced at an almost glacial pace. As such, we are still a long way off using batteries for heavy-duty use such as in transport. Sure, there are cars such as the Tesla of the Chevy Volt that are battery powered, but there are still issues with the weight and safety (and cost!) of many rechargeable systems. For example, 1 litre of petrol still has a higher energy capacity that the Li ion batteries that your laptop or mobile invariably use.[1]
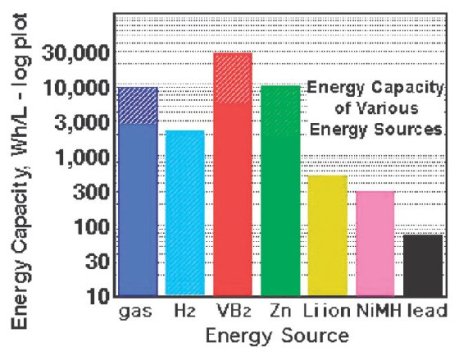
Copyright of the Royal Society of Chemistry
Nothing beats petrol, litre for litre, that we can easily use.
Except, you will notice from the above image that there is something that has a higher energy capacity. Stuart Licht and colleagues at MIT have developed an electrochemical system that is based on air and borides, in their particular case, vanadium boride.[1]
As Licht himself says in his article, air batteries are nothing new – Zn/air batteries were first reported in 1932.[2] Nonetheless, it is only comparatively recently that air batteries have started to receive serious attention. The work of STAIR springs to mind. It stands to reason that if half of your battery “runs” on air, then your cost and weight drop – you do not have to carry around as much of the chemicals you might originally have required.
Licht’s work shows substantial promise. He has developed a system with a higher capacity than petrol based on vanadium boride despite the fact that “basic physical chemical properties of VB2 are scarce.” There is much scope for improvement on something which is already an excellent start.
Can we improve the vanadium boride?
Can we find a low-temperature/”easy” way of preparing vanadium boride?
Are there better materials?
We shall see.
![]() [1] Licht, S., Wu, H., Yu, X., & Wang, Y. (2008). Renewable highest capacity VB2/air energy storage Chemical Communications (28) DOI: 10.1039/b807929c
[1] Licht, S., Wu, H., Yu, X., & Wang, Y. (2008). Renewable highest capacity VB2/air energy storage Chemical Communications (28) DOI: 10.1039/b807929c
[2] Kinoshita, K, Electrochemical Oxygen Technology, Wiley-Verlag VCH, Weinheim, Germany, 1992, pp. 259-260.
Photocatalysis: Buy my snake oil September 30, 2009
Posted by calvinus in Energy, Green Chemistry, Photocatalysis.Tags: Energy, Photocatalysis
3 comments
Was at a one-day meeting the other day. There were couple of companies trying to sell themselves and their products. Photocatalysis was mentioned a couple of times in despatches.
Photocatalysis, for those that do not know, is a way of using light (usually sunlight) to drive a “useful” chemical reaction. In the case below, this could be splitting water into oxygen and hydrogen, but it might also involve turning organic chemicals into water and carbon dioxide.

I should say that it is telling that I see this figure appear quite often but I do not know who was the originator of the image.
Normally the photocatalyst is a semiconductor and the vast, vast majority of scientific articles published use nanoparticles of titanium dioxide to do this. The popularity of titanium dioxide is partly historical (Fujishima and Honda discovered the phenomenon using titanium dioxide[1]) but mostly due to titanium dioxide’s efficiency at converting light that has enough energy to a chemical product.
And therein lies an important drawback: the light must have enough energy to overcome barriers in the semiconductor. For titanium dioxide, this means the light has to be particularly high in energy – it can only use ultraviolet light. Given that this is only around 4% of natural sunlight, this is a problem if photocatalysis is to be a useful process. This is especially the case for indoor applications where room lights tend to have negligible ultraviolet light.
There are, it would appear, companies out there that claim to be able to do everything through photocatalysis. They can:
- destroy nasty pollutants in water
- make glass, concrete, roads, buildings that clean themselves
- kill bacteria such as “E. coli, MRSA, swine flu, bird flu”
- destoy spores and turn them into carbon dioxide (!)
Some of the claims are fanciful, to say the least, but there are some basis in these claims. Destruction of organic pollutants has been known for a while and substantially researched. Self-cleaning glasses are known (see St Pancras station for an example of their use). Anti-bacterial propoerties are again reported in the scientific literature although the jury is out – I don’t believe anyone fully understands the possible mechanism and I have certainly NEVER seen any reports of photocatalysis being used to combat the bacteria du jour, swine flu or bird flu. You might, if you were being charitable, sugest that these claims may have been the result of someone not fully understanding the background science.
Maybe.
However, what triggers my abject cynicism is that one company went as far to say that their photocatalysts worked in both outside applications AND indoors. When asked how the performance compared, indoor and out, I was very surprised to hear them openly admit that they hadn’t actually tested it. So how could they make these claims? “Er…”
This narks me. This narks me a lot. Not only does it border on fraudulent, it does give the whole area an air of “buy my snake oil”. For those who are trying to do good, honest work in this area, it pollutes the pool of potential investors and cheapens what could be a useful area of renewables research.
Of course, preying on people’s fears (“Kills MRSA and Swine Flu dead”) is one thing, but would they use the same bombast if they were a pharmaceutical company and had people’s lives hanging on their results?
![]() [1] Fujishima A, & Honda K (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature, 238 (5358), 37-8 PMID: 12635268
[1] Fujishima A, & Honda K (1972). Electrochemical photolysis of water at a semiconductor electrode. Nature, 238 (5358), 37-8 PMID: 12635268
Talking of Cold Fusion April 13, 2009
Posted by calvinus in Cold fusion, Physics.Tags: Cold fusion, Energy, Physics
2 comments
I previously mentioned cold fusion, my memory being given a bit of a kick by recent events at an American Chemical Society meeting. Good to see people are still plugging away at the less fasionable (to say the least) areas of research. Cold fusion still on the agenda, even if it is not still called that for fear of inviting accusations of quackery.
Many thanks to the New Scientist for digging out the original press conference video.
Beware the perils of publishing hot (or cold) science through routes that are not peer reviewed! Bloggers beware.
