Compare the Materials.com October 24, 2010
Posted by calvinus in Meerkat Science.Tags: Meerkat Science
add a comment
I now have a new hobby. Spotting tortured English (Meerkatish?) in published articles. First up, from Solar Energy, 2006, 80, 1098:1
Here the samples’ simples’ flat band potential, which is determined by a potential of the Nb2O5 conduction band, is rather negative and, it is additional bias from an external source not be necessary for realization of water photoelectrolysis.
From the same article:
The focus of this paper is investigations of the photoelectrochemical properties of polycrystalline photoelectrodes…and to study the structure of the metal oxide semiconductor–electrolyte interface by via impedance spectroscopy.
Don’t even sound same?
Now, I do not want to criticise (or criticize) the standard of English of the authors who are having to overcome being forced to communicate their work in a language other than their own. I know my own prose is usually strewn with typos and sentences that start with prepositions. But given how much publishers charge for journal subscriptions and access, you might think that they could at least polish the language of their product a little. You might think that they could afford it – they are, after all, saving a tidy sum by not paying highly qualified referees and authors! Who knows, they may even be saving money on cheap deals on their car insurance too.
Simples!
![]() [1] Aroutiounian, V., Arakelyan, V., Shahnazaryan, G., Stepanyan, G., Khachaturyan, E., Wang, H., & Turner, J. (2006). Photoelectrochemistry of semiconductor electrodes made of solid solutions in the system Fe2O3–Nb2O5 Solar Energy, 80 (9), 1098-1111 DOI: 10.1016/j.solener.2005.10.005
[1] Aroutiounian, V., Arakelyan, V., Shahnazaryan, G., Stepanyan, G., Khachaturyan, E., Wang, H., & Turner, J. (2006). Photoelectrochemistry of semiconductor electrodes made of solid solutions in the system Fe2O3–Nb2O5 Solar Energy, 80 (9), 1098-1111 DOI: 10.1016/j.solener.2005.10.005
Wavelength to electronvolts October 17, 2010
Posted by calvinus in Energy, Spectroscopy.Tags: Spectroscopy
add a comment
I have forgotten how many times I have forgotten the automatic reflex that used to be quick conversion between various units of energy, wavelength and wavenumber. The latest bout of amnesia has prompted E=hν
c=λν
E=hc/λ
and
λ=hc/E or λ=1240/E if E is in eV
There is also a nifty wee website that converts all of the spectroscopic terms that you could ever hope to shake a stick (or rigid rotor) at.
I will forget again, I know that for a fact, but the above link means that having measured the diffuse reflectance spectrum of his latest semiconductor, my student can then convert his optical band gap data into eV or kcal mol-1. And now that he has done that, I might ask him to express it all in Hartrees or ergs…
Stop Me If You Think You’ve Heard This One Before September 25, 2010
Posted by calvinus in Teaching, The Academic Life.Tags: Teaching, The Academic Life
add a comment
Summer holidays? What are they, you will have to remind me? Something is wrong when you actually look forward to the students come back as it brings with it a hint of normality (only just a hint, mind) and a slackening of workload!
This summer I have had to supervise/run/deal with 18 project students (not all my own). Not all passed, some may even have been stupid enough to steal some material from published work.

How often do you have to repeat yourself?
Do not plagiarise. Plagiarism is theft.
Do not copy form books/journals/tinterweb. Plagiarism is theft.
Do not plagiarise, you will fail.
So…lets say you have to hand in a project report and it is found to contain 67% of thefted material, lets say you have thefted 42% from one source.
How to deter students plagiarising? Partly this is cultural – many of our students are used to rote learning, many are not used to putting things in their own words, many leave things to the last minute and obey Hess’s law to submit any old tosh for the deadline.
How to deter students plagiarising?
This is doing my head in. Spend 6 months trying to get someone through a project only for them to hand in a regurgitated version of wikipedia gets to be a little tiring. My first reaction when marking plagiarised work is to wince. It’s painful to discover you have wasted your time.
“And the pain was enough to make
A shy, bald, buddhist reflect
And plan a mass murder
Who said I’d lied to her?”
S.P. Morrissey, 1987
The student still doesn’t know she is being done for plagiarism. That will come. Another fun day of interviewing thieves awaits. Joy of joys.
Norfolk, Egypt and Guernica May 30, 2010
Posted by calvinus in Birding, Music.Tags: Birding, Music
add a comment
I went to Chatteris AND saw a linnet.
It all makes sense, really it does. Somehow, during the madness of revision and exam seasons I managed to sneak away for a short break to Norfolk. A propos exam season, why do I have tutees that think that they can wander into my office, ask me questions about a subject on which I do not teach, nor know anything about, and expect that the pearls of wisdom I cast before the swine will get them through the exam they have in two hours time? I like their flattery, but not their delusions.
Anyhow, it was a welcome break. Ostensibly it was to watch an ex-Velvet recreate Paris 1919 – Endless Plain of Fortune and Half Past France complete with strings were both breathtaking. Nevertheless, I must be getting old. Norfolk offers some good birding locations and I managed to catch a bit of twitching on the side. Whilst out on the broads, there were greylag geese aplenty. It was also the first time I have (knowingly) seen an egyptian goose in the wild. These are ugly beasts.

The egyptian goose is not a goose, but a shelduck (and there were plenty of those too). Whomsoever brought these things to the UK had a taste that can be described as doubtful. That said, having watched them, and heard them, they reminded me of something. W.N. Herbert wrote excellent The Guernica Duck, presumably not after a weekend in Wroxham, but there is a passing similarity.
“Look at me: I’m frightened too!”
Taking an Artificial Leaf Out of Nature’s Book March 13, 2010
Posted by calvinus in Energy, Nano, Renewables, Solar.Tags: Energy, Nano, Renewables, Solar
add a comment
Solar cells are common enough. Small solar cells are found in a variety everyday applications. The normal example I give is in calculators although it looks like these are becoming less and less common if my students are anything to go by – many of my first years don’t own a “proper” calculator – iPhones are the order of the day. However, plonk a solar cell on the side of a building and we have an additional problem. Buildings tend not to move, but the sun does which means you are limited practically to south-facing surfaces. Now, a full GIS study of how to maximise the power harvested by solar cells in a built-up area would help overcome some of this problem, but it doesn’t solve another issue. Dirt!
Would you eat your dinner off the street? Stick something in an urban environment and you will inevitably get all manner of dust, detritus and general keech stuck to it. A layer of grime on your shiny solar cells means light will not make it through to the photovoltaic underneath, which means a less efficient solar cell. Yi Cui at Stanford University has come up with an elegant way around this dirty issue.

What gives lotus leaves their water repelling properties are tiny spikes along the surface of the leave which stops water droplets from wetting the surface. Given that most dust particles are deposited on glass surfaces after rainfall, Cui figured it would be useful to put this to good effect in solar cells. By pattering the amorphous silicon that solar cells are often made from in a way that mimics the lotus leaf, water droplets can run off the surface taking any dirt with them, rather than sticking around and evaporating (which would leave the dirt behind)[1]
deposited on glass surfaces after rainfall, Cui figured it would be useful to put this to good effect in solar cells. By pattering the amorphous silicon that solar cells are often made from in a way that mimics the lotus leaf, water droplets can run off the surface taking any dirt with them, rather than sticking around and evaporating (which would leave the dirt behind)[1]
There is an additional, more fundamental benefit to this type of patterning. This films of amorphous silicon tend to be pretty flat. They also have a habit of behaving a little too much like a mirror than is useful for a solar cell. If you absorb light, you generate electricity; if you reflect light, like a mirror, you lose efficiency. The roughened “domed” structure means that less light is reflected. Cui saw this in their results – their rough structured cells were found to be 5.9% efficient, which is better than the 4.7% efficiency for flat, smooth surfaces. Sometimes it pays to take the rough with the smooth.
![]() [1] Zhu, J., Hsu, C., Yu, Z., Fan, S., & Cui, Y. (2009). Nanodome Solar Cells with Efficient Light Management and Self-Cleaning Nano Letters DOI: 10.1021/nl9034237
[1] Zhu, J., Hsu, C., Yu, Z., Fan, S., & Cui, Y. (2009). Nanodome Solar Cells with Efficient Light Management and Self-Cleaning Nano Letters DOI: 10.1021/nl9034237
Cold, Rain and Snow (but Mostly Snow and Ice) January 10, 2010
Posted by calvinus in Physical Chemistry.Tags: Physical Chemistry, The Fall, Weather of Malcontents
2 comments
This is the spring without end
This is the summer of malcontent
This is the winter of your mind
M. E. Smith, 1992
Actually Mark, this is the Winter of Malcontents. In case you had failed to notice, the Angles are unhappy with the recent weather that has afflicted Albion’s Plain.

A satellite image of the UK taken from NASA's TERRA satellite

A satellite image of NASA's TERRA satellite taken from the UK.
All this snow and ice is all a bit of a frightful inconvenience, quite frankly. I can’t get my car out of the drive for all of these roughians parking in the middle of the road, just abandoning their cars willy-nilly.
Get over it. It’s cold, it’s slippy. Dress accordingly. Act sensibly.
Councils are running out of salt to spread on the road and to a certain extent, I can understand this. The weather has been a freakish, I mean how often in the last few years has Bromley (for example) had to thole this much snow. And so, in times of hardship, it is right that councils are rationing their supplies of salt. I have heard the odd (and it is odd) criticism of councils that won’t go out and salt the roads and pavements when it gets too cold. “They should have been out…saying that the salt won’t work at -6°C is just an excuse.” Err…actually…it is standard physical chemistry, my friend.
The water molecules in ice crystals are held together nice and tightly in a rigid structure that looks not unlike a honeycomb. Voilà:

The structure of water moleculs in ice.
In a liquid, the water molecules are pinging around moving all over the place, they are not as tightly bound to each other as in an ice crystal. As the temperature drops, the movement of the water molecules slows down and this is what allows ice to form this regular structure. If you add something like salt into this mix, the atoms in salt disrupt the regular arrangement of water molecules above. When this disruption happens, the water molecules find it easier to move and break free, thus melting the ice that had formed. If you want a nice animation of what happens when water freezes (at a molecular level) clicky here.
However, this only works until the temperature gets cold enough again to slow down the water molecules enough to allow them to be stuck back into the ice crystal. What temperature this happens at depends on the nature of the salt (such as what size the atoms are, the atoms present, etc.) and how much you use. Sodium chloride and calcium chloride are commonly used salts for icy roads. The Finns, rather typically given their climate, have been looking for alternative salts and thought about using potassium formate[1] (although a lot of ants might be needed to keep Finnish roads gritted with this!).
Standard table salt can be effective at temperatures as low as -21°C if you use enough of it. Unfortunately this is at such high quantities of salt that I suspect we will all start complaining about how much our cars are rusting…
![]() [1] Hellstén PP, Salminen JM, Jørgensen KS, & Nystén TH (2005). Use of potassium formate in road winter deicing can reduce groundwater deterioration. Environmental science & technology, 39 (13), 5095-100 PMID: 16053115
[1] Hellstén PP, Salminen JM, Jørgensen KS, & Nystén TH (2005). Use of potassium formate in road winter deicing can reduce groundwater deterioration. Environmental science & technology, 39 (13), 5095-100 PMID: 16053115
A thousand cuts: flaying higher education starts here. December 23, 2009
Posted by calvinus in The Academic Life, Training.Tags: Academics, Government, Universities
2 comments
And so it begins.
Universities funding cut by £533m for higher education.
I know this is inevitable and it isn’t as large a cut as I first feared but it is only the first. There will be many more. However, it isn’t the size of the cut that worries me, nor is it the fact that the information has crept out when the country is worrying about the weather and getting back for Christmas. What worries me more is the following,
The government also wants to see more degrees completed over two years rather than three as a way of easing the funding crisis and to broaden education to a wider range of students.
This would tend to appeal to those doing more vocational subjects such as engineering and law.
More degrees to be completed over two years, rather than three? That is not a degree. The three-year degree is bad enough, and rather typical of the English education system in that it is far, far too focussed and results orientated. Spitting people out of the system with a two-year degree, most likely a foundation degree by the looks of things is just a way of keeping young people in a system, off the statistics sheets for as long as possible. 2 year “vocational subjects”. Be honest. Invigorate some form of apprenticeship programme that doesn’t involve Siralan, you muppet. Universities are not the place to that. Of course, it will help you reach the target of 50% of da yoof in higher education. “Higher” than what, remains to be seen.
I am, sadly, resigned to very hard times ahead for the HE sector, especially at “widening participation” institutions such as my own. I am resigned to the cuts in funding, I am resigned to the massive spike in workload and the job insecurity. Don’t make matters worse than they already by adding insult to injury with bizarre, illogical directives that will obliterate any semblance of quality in an already stressed system. I have no idea what will be left of the HE sector by the end of this.
Mathematically Safe (falling off a log) December 12, 2009
Posted by calvinus in The Academic Life.Tags: Maffmaticks, Teaching
add a comment
Two first year students were in my office yesterday. We were going through an impromptu tutorial on UV/vis and the Beer-Lambert law (or Beer-Bouguer law if we are to acknowledge the actual originators). Calculators were out and it would appear that there is some difficulty calculating absorbance (A) from transmittance (T):
A = -log10(T)
A simple enough equation for a first year student you might think, but once we got over the problem of logarithms, we struck on the increasing problem of how to use a calculator. Both students are studying life science degrees and one suggested that we should teach maths classes. Now, I happen to agree fully with this and am not entirely sure why we don’t (at least, not sure enough to comment openly here). Unfortunately, what the student had in mind was not teaching actual maffmaticks, but use of calculators as what followed was a 15 minute “masterclass” in using the [shift] button to go from the log of 1000 being equal to 3 and back again.
Looks like we are not alone in this as the following image came from the website of The Pharmacy Examining Board of Canada:

I realise, or course, that this is a common problem for those of us at the frontline of “widening participation” institutions such as mine, but it will get worse. How many of “da yoof” use/own calculators these days? Not that many. I’ve lost count of the number of times that I have narked at people using mobile phones in the lab, not for communication, but for working out how many moles of hydrochloric acid are in a 25 ml sample!
Exponentials? There’s an app for that, innit?
Recharge Your Batteries November 15, 2009
Posted by calvinus in Batteries, Energy, Physical Chemistry, Renewables.Tags: Batteries, Energy, Physical Chemistry, Renewables
2 comments
![]() Every so often, you come across an article that looks really interesting. Interesting in a good way . Often you get the chance to review manuscripts that are “interesting”, but this is not one of those.
Every so often, you come across an article that looks really interesting. Interesting in a good way . Often you get the chance to review manuscripts that are “interesting”, but this is not one of those.
I was actually looking for something else and in the process of re-establishing some order to the chaos, this Chemical Communications article popped up from the pile. Renewable electrochemical systems, i.e., rechargeable batteries, have long undergone development and if I compare my first rechargeable batteries with the lifetime of the battery in the laptop I am using at the moment, the difference is night and day. Having said that, such efficiencies may partly bedown to better power management. The underlying technology is not that radically different. As Jean-Marie Tarascon commented at a meeting at the Royal Society in London at the start of this year, rechargeable battery research has advanced at an almost glacial pace. As such, we are still a long way off using batteries for heavy-duty use such as in transport. Sure, there are cars such as the Tesla of the Chevy Volt that are battery powered, but there are still issues with the weight and safety (and cost!) of many rechargeable systems. For example, 1 litre of petrol still has a higher energy capacity that the Li ion batteries that your laptop or mobile invariably use.[1]
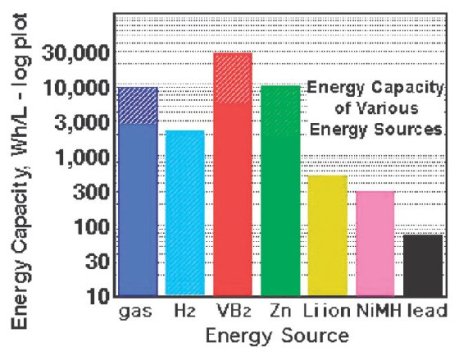
Copyright of the Royal Society of Chemistry
Nothing beats petrol, litre for litre, that we can easily use.
Except, you will notice from the above image that there is something that has a higher energy capacity. Stuart Licht and colleagues at MIT have developed an electrochemical system that is based on air and borides, in their particular case, vanadium boride.[1]
As Licht himself says in his article, air batteries are nothing new – Zn/air batteries were first reported in 1932.[2] Nonetheless, it is only comparatively recently that air batteries have started to receive serious attention. The work of STAIR springs to mind. It stands to reason that if half of your battery “runs” on air, then your cost and weight drop – you do not have to carry around as much of the chemicals you might originally have required.
Licht’s work shows substantial promise. He has developed a system with a higher capacity than petrol based on vanadium boride despite the fact that “basic physical chemical properties of VB2 are scarce.” There is much scope for improvement on something which is already an excellent start.
Can we improve the vanadium boride?
Can we find a low-temperature/”easy” way of preparing vanadium boride?
Are there better materials?
We shall see.
![]() [1] Licht, S., Wu, H., Yu, X., & Wang, Y. (2008). Renewable highest capacity VB2/air energy storage Chemical Communications (28) DOI: 10.1039/b807929c
[1] Licht, S., Wu, H., Yu, X., & Wang, Y. (2008). Renewable highest capacity VB2/air energy storage Chemical Communications (28) DOI: 10.1039/b807929c
[2] Kinoshita, K, Electrochemical Oxygen Technology, Wiley-Verlag VCH, Weinheim, Germany, 1992, pp. 259-260.
Raman Spectroscopy: As Seen in CSI? October 11, 2009
Posted by calvinus in Analytical Chemistry, Forensics, Raman, Spectroscopy.Tags: Analytical Chemistry, Forensics, Raman, Spectroscopy
add a comment
A recent editorial in Analytical Chemistry[1] alerted me to a paper that had initially slipped under the radar.
Kelly Virkler and Igor Lednev[2] were able to determine the difference between dried traces of human, cat and dog blood using a combination of lasers and computer analysis. This is quite impressive. The technique they used, Raman spectroscopy, is a notoriously weak technique.
In Raman spectroscopy, a sample is illuminated with a laser and miniscule changes in the colour of light that is scattered from the sample contains valuable information about molecules present in the sample. These are very, very small changes. Furthermore, most of the light that is scattered does not contain this information, only 1 in a million does. Nonetheless, the use of lasers means that Raman spectroscopy lends itself to the analysis of microscopic samples as you can feed your laser beam down a standard microscope. Furthermore, you don’t need to prepare or alter your samples in any way, although firing high powered lasers at something does tend to fry your samples if you are not careful – it can easily be a non-destructive technique.
What is most impressive about the work is the fact that they can not only determine that an unknown dried sample sitting at a crime scene is that of blood, but also that they can discriminate between different species that this blood might have come from.

If you look at the raw data you would normally get from Raman spectroscopy you might think that there is little difference between results. To the human eye, the Raman spectra of human blood, feline blood or canine blood look very similar. Use of computer analysis allowed the authors to tease out the subtle differences that are imperceptible to the eye.
If you dug deeper, you might even find consistent differences between different human samples. Has the sample come from a man or a woman? Were they healthy or did they have a particular illness? Now that would be very useful for forensics.
![]() [1] Gebel, E. (2009). Species in a snap: Raman analysis of blood Analytical Chemistry, 81 (19), 7862-7862 DOI: 10.1021/ac901827u
[1] Gebel, E. (2009). Species in a snap: Raman analysis of blood Analytical Chemistry, 81 (19), 7862-7862 DOI: 10.1021/ac901827u
![]() [2] Virkler, K., & Lednev, I. (2009). Blood Species Identification for Forensic Purposes Using Raman Spectroscopy Combined with Advanced Statistical Analysis Analytical Chemistry, 81 (18), 7773-7777 DOI: 10.1021/ac901350a
[2] Virkler, K., & Lednev, I. (2009). Blood Species Identification for Forensic Purposes Using Raman Spectroscopy Combined with Advanced Statistical Analysis Analytical Chemistry, 81 (18), 7773-7777 DOI: 10.1021/ac901350a